Oceans, Ice, and Rocks
ACS Climate Science Toolkit
The unique properties of water, due to its molecular structure and hydrogen-bonding capacity, are important to climate science. Water exists in all three phases at the temperatures on the surface and in the atmosphere on Earth. Ice floats on liquid water, so only ocean and lake surface waters freeze, not the bulk of the liquid. The liquid’s high heat capacity makes the oceans an enormous energy reservoir that dampens diurnal and seasonal swings in temperature. Water is an excellent solvent for many ions and polar molecules, especially those with hydrogen-bonding capacity. The ability of water molecules to both accept and donate protons makes it the central actor in aqueous acid-base reactions.
The other modules in this ACS Climate Science Toolkit deal almost entirely with the Earth’s atmosphere—its controls on Earth’s climate, the impact of human activities on its properties, and, hence, their impact on the climate, especially the temperature. This emphasizes the most direct effect of human activities—increasing the atmospheric concentrations of greenhouse gases, which upsets the energy balance of the planet by decreasing the amount of thermal IR radiation leaving the top of the atmosphere.
But it cannot be only the atmosphere that warms as the energy balance is upset. Since 70% of the Earth’s surface is water, the oceans absorb the majority of the incoming solar energy. If they are not losing energy as rapidly as in the past, oceans are warming. With their high heat capacity, oceans do not warm as rapidly as the atmosphere, but store large amounts of energy. As water warms, it expands, so the volume of the warming ocean increases, producing rising sea levels.
Some of the ocean surface as well as some land surface is covered with ice, which can also absorb energy directly from the sun as well as from a warmer atmospheric or ocean environment. The absorbed energy can be taken up in the solid-to-liquid phase change, so the energy imbalance results in melting of sea ice and land ice—glaciers and snow pack. Melting land ice contributes to rising sea levels.
The other modules give only a cursory look at the ultimate fate of the CO2 that human activities have released to the atmosphere. To complete the picture, we have to account for the interactions between the atmosphere and the surface of the Earth, its oceans, crust, and biosphere. These are the components of the system we live in and experience most directly. They comprise a thin outer layer of the planet that contains almost all of Earth’s carbon in the form of carbonate rocks. Exchanges among the various components depend on the chemistry and biochemistry of CO2 and are sensitive to changes in its concentration.
Of particular importance, CO2 has a rich chemistry as a solute in the oceans. The shifts in equilibrium concentrations among carbonate, bicarbonate and hydrogen ions, as more CO2 dissolves, are part of climate science that has important consequences for marine ecosystems and geology. Marine organisms use calcium and other mineral ions together with carbonate to build shells and cell walls that end up as sediments on the ocean floor. Over geological time and metamorphic transformation the mineral ions in these sediments can end up on the surface as silicate rocks. Weathering of silicate rock by CO2 dissolved in rainwater and streams, then returns minerals to the oceans to continue the process as part of Earth’s carbon cycling. Climate chemistry takes place on time scales from “instant” acid-base reactions to millions of years for geological processes.
Changes in the atmosphere and oceans are intimately entwined with one another. Tropospheric winds push vast quantities of seawater around the ocean basins, transferring energy and solutes across the globe. As water evaporates over warm ocean areas it condenses to form clouds that the atmosphere carries to cooler areas where the water can precipitate to complete the water cycle. Understanding ocean-atmosphere interactions is important for mitigating or adapting to their effects.
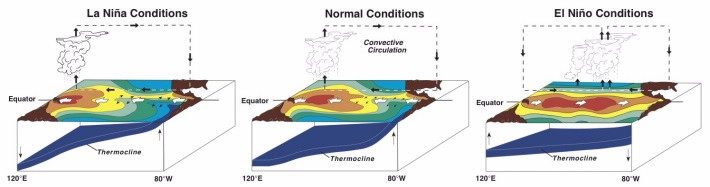
For example, among the most well studied, but still not completely understood, of these phenomena are the El NiÑo Southern Oscillation (ENSO) events represented schematically in the diagrams below. ENSO events are synergistic oceanic (El Nino) and atmospheric (Southern Oscillation) changes that influence temperatures and weather patterns across most of the planet. ENSO events are semi-periodic changes in the surface temperatures along a swath of the Pacific Ocean that straddles the equator and extends from the coast of South America to the western Pacific. The eastern part of the swath warms during an el NiÑo event and cools during a la NiÑa. The warming results from a weakened upwelling of deep cold water and the cooling from an enhanced upwelling of deep cold water. Learn more about the ENSO and its effects worldwide.
The Earth’s atmosphere extends a few tens of kilometers above the surface and the crust a few tens of kilometers below the surface. By comparison, if an egg were the size of the Earth, its shell would be about three times as thick as Earth’s fragile atmosphere-crust shell.
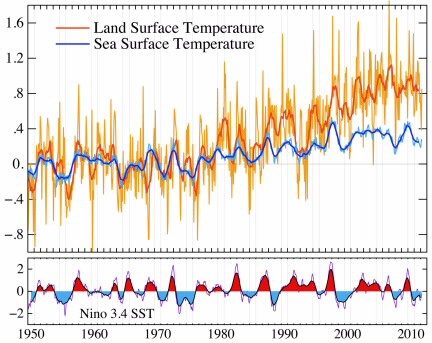
ENSO conditions and their consequences are well characterized. The lower part of the figure below shows that el NiÑo and la NiÑa events recur periodically, but irregularly, and last for relatively short lengths of time. The events are extensive enough to leave their fingerprints on the average conditions of the planet. You can see that there are ripples in the global data that correspond to many of the ENSO events.
This figure shows the correlation between global temperature anomalies and the temperature anomalies associated with the ENSO in the warm eastern Pacific part of the el Niño and, hence, in the cool part of the la Niña. The global data are from the GISS temperature pages and the ENSO data are from the NOAA Climate Prediction Center.
Although the ENSO is not responsible for net global warming, the possible effects of warming oceans and atmosphere on the ENSO itself are unknown. Because the ENSO has a strong influence on where and how powerfully winds blow and where precipitation does or does not occur, understanding its behavior could be important for adaptation to the climate of a warming world.
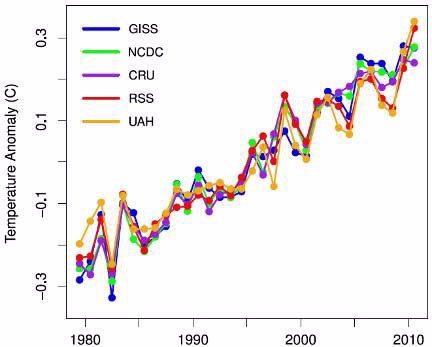
The five data sets plotted here are based on global surface or lower tropospheric temperature anomalies from the climate science groups noted. For each one, statistical analyses were used to remove the quasi-periodic effects of ENSO events, the transient cooling effects due to atmospheric aerosol particles from volcanic eruptions, and the variation due to the 11-year solar cycle.