A Multilayer Atmosphere Model
ACS Climate Science Toolkit | How Atmospheric Warming Works
A single layer, grey-body atmosphere model for the Earth and its atmosphere illustrates the fundamental mechanism for radiative planetary atmospheric warming. It shows that there are both surface and atmospheric sources of the infrared emissions leaving the planet. However, we know that the atmosphere is not all at the same temperature and that it does not absorb and emit radiation as an ideal grey body with the same emissivity at all wavelengths. A first modification of the model is to introduce the atmospheric temperature profile.
Atmospheric temperature profile
A physical consequence of the warmed surface of the Earth is warming of the atmosphere near the surface by conduction. As it warms, the gas expands, becomes less dense, and is buoyed up from the surface by convection. As the gas rises, it expands further because atmospheric pressure decreases with altitude. The expansion requires the gas to do work against the surrounding atmosphere, thus cooling the gas as it rises higher into the atmosphere.
If the atmosphere contains water vapor, the cooling expansion can cause the water vapor to condense at higher altitudes, forming clouds of tiny water droplets. Condensation releases the energy, often called latent energy, that was required to vaporize the water at the surface and adds energy to the atmosphere. The effects of these conduction, convection, evaporation, expansion and condensation processes are part of Earth’s energy balance. These processes produce the movement of gases in the lowest layer of the atmosphere that are responsible for winds and weather and give rise to its name, troposphere, from the Greek tropos, mixing or turning.
Adiabatic cooling is the temperature change when a volume of gas at a higher pressure expands against a lower pressure to a new larger volume at the lower pressure. The amount of cooling for a dry sample can be calculated from the constant pressure and constant volume heat capacities of the expanding gas. If the gas contains water vapor, the water vapor could condense as the gas cools and release energy that decreases the amount of cooling.
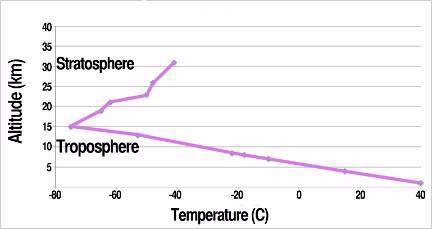
These processes also produce the temperature profile for the troposphere—temperature decreasing with altitude—illustrated in this figure. The observed change of temperature with altitude is called the lapse rate, which varies from about 4 to 10 K·km–1 over different parts of surface. For example, the top of the troposphere is at an altitude of about 16 km in the figure and the atmospheric temperature at that altitude is colder than at the surface by about 110 K (or °C), so the lapse rate is -(-110 K)/(16 km) ≈ 6.9 K·km–1.
Although the temperature of the troposphere decreases with altitude (h), the lapse rate has a positive sign, because it is defined as -dT/dh.
The figure shows the observed atmospheric temperature as a function of altitude over Tucson, AZ, in late afternoon, 14 August 2000, when the surface temperature was 36.7 °C.
At some altitude, the convection processes driven largely by energy transported from the surface finally reach a limit and the atmospheric temperature stops dropping. This limit, the top of the troposphere, is higher in the warm-surface tropics, up to about 20 km, than over cold-surface polar regions, about 7 km. If there were no sources of energy above the limiting altitude, the atmospheric temperature would remain roughly constant or drop slowly with increasing altitude.
However, in Earth’s oxygen-containing atmosphere, ultraviolet (UV) radiation from the sun drives photochemical reactions whose net result is to add energy to the atmosphere above the troposphere. At these high altitudes, ozone, O3, is formed and decomposed by high and medium energy UV radiation, respectively. These are the processes that protect life on the planet by absorbing these damaging UV wavelengths and at the same time warming the surrounding atmosphere, so that temperatures increase with altitude, as shown in the figure. Because warmer, less dense gas is layered over cooler, denser gas, there is little vertical mixing and this part of the atmosphere is stratified—hence the name stratosphere.
A multilayer, non-grey-body troposphere model
The data in the figure show a sharp reversal in the slope of the temperature at about 16 km altitude, which implies a rather sharp boundary between the troposphere (temperature decreasing) and stratosphere (temperature increasing). Under other conditions the boundary is not so distinct and there is an atmospheric layer called the tropopause where the temperature is about the same as at the top of the troposphere. The tropopause ends when the temperature begins to increase, which defines the stratosphere.
A model, shown below, that accounts for the decreasing temperature and pressure as the altitude increases, is a troposphere made up of a stack of multiple layers of gas of decreasing temperature and pressure. Within each layer the temperature and pressure are constant and the gases are assumed to be in thermal equilibrium and in equilibrium with respect to absorption and emission of thermal infrared radiation, just as in the single-layer, grey-body atmosphere model. Under these conditions, the thermal IR energy emitted from each layer is governed by the Planck black-body function – a function of the emission wavelength and the temperature of the layer.
If these atmospheric layers were composed entirely of nitrogen, oxygen, and argon—all non-IR absorbing gases—the emissivity and absorptivity in the thermal infrared spectral region would be essentially zero at all wavelengths and a grey-body model would be satisfactory. The presence of trace amounts of IR-absorbing greenhouse gases introduces wavelength regions with higher atmospheric emissivities and absorptivities, so the grey-body assumption of constant emissivity is not valid. Instead, we have to use the Planck black-body function and account for emissivity and absorptivity at individual wavelengths (or frequencies).
A gas such as CO2 that absorbs and emits thermal IR radiation remains in thermal equilibrium within an atmospheric layer by collisional exchange of energy with the other gases. A CO2 molecule that absorbs energy to excite its vibrational motion very quickly loses the excess energy in collisions and has a warming effect on the layer. Another CO2 molecule that has a high enough energy (within the temperature-dependent Maxwell-Boltzmann distribution of energy in the layer) can become vibrationally excited and emit energy in the thermal IR. Collisions soon bring this molecule back into thermal equilibrium and have a cooling effect on the layer. The combination of these two processes occurring at equal rates, equal emissivity and absorptivity, keeps the absorbing gas in thermal and radiative equilibrium within the layer.
The multilayer atmosphere model is illustrated here for a three-layer atmosphere. The radiation represented in the diagram is at a wavelength that can be absorbed by an IR-active molecule in the atmosphere. The squiggly arrows represent radiation emitted by the body from which the arrow originates. The straight-line arrows represent radiation that has been transmitted without absorption through a layer. The temperature-dependent Planck black-body function for this wavelength is represented by B with a subscript denoting the temperature of the layer to which it applies. The emissivity of each layer is similarly labeled. The temperatures are TP at the surface and T1 > T2 > T3 for the temperatures of the layers. Decreasing pressure and, hence density and partial pressure, of the atmospheric gases is represented by the decreasing depth of color of the layers.
To interpret this model, begin with the radiation from the Earth’s surface at the bottom left of the diagram. Since the surface emits like a black body, the energy emitted at the wavelength in question is the emission for this wavelength from a black body at the temperature of the surface, BP. When this radiation interacts with IR-absorbing molecules in layer 1, some of it is absorbed, ε1BP, and the remainder, (1 – ε1)BP, passes through, as shown by the straight arrow emerging from the layer. The absorbed energy is not labeled in the diagram, because the focus of the model is on the radiation that has to leave the atmosphere in order to maintain (or try to maintain) the energy balance of the planet.
Radiation from the surface and each layer is, of course, emitted in all directions. In this one-dimensional model, emission is shown only up and down relative to the Earth’s surface.
When the radiation transmitted through layer 1 interacts with the IR-absorbing molecules in layer 2, some of it is absorbed, ε2(1 – ε1)BP (not labeled), and the remainder, (1 – ε2)(1 – ε1)BP, passes through. This process is repeated in layer 3, so the atmospheric layers have attenuated energy that started from the surface and only (1 – ε3)(1 – ε2)(1 – ε1)BP leaves the top layer. Similarly, radiation emitted upwards from any lower layer leaves the atmosphere from the top layer after attenuation by the intervening layers. From the point of view of an observer outside the atmosphere, all the radiation at this wavelength is seen as coming from the topmost, coldest atmospheric layer.
The absorptivity (and therefore the emissivity) of the layers decreases with altitude for several reasons, the most obvious being the decrease in numbers of IR-absorbing molecules as the density of the atmosphere decreases. Emissivity is also temperature dependent, decreasing with decrease in temperature. Finally, we must account for the effects of collisional absorption-emission line broadening. Perturbation of the energy of an absorbing or emitting molecule during a collision with another molecule leads to a broader spread of absorbed or emitted wavelengths than for the undisturbed molecule. Any collision can cause this perturbation, so the amount of the line broadening is a function of the total pressure or density of the gas, including both IR-absorbing and non-absorbing molecules. Thus, line broadening for IR-absorbing gases is larger at lower altitudes and decreases with altitude. A wavelength that is absorbed relatively strongly in the broadened wavelength range at lower altitudes will absorb more weakly at higher altitudes where the broadening is less. Thus the absorptivity and emissivity at this wavelength will decrease with altitude.
Application to Earth’s Atmosphere applies this analysis of a three-layer atmosphere to observations of the Earth’s actual atmosphere.
The atmosphere of Mars, at a total pressure of 0.64 kPa (about 1/160 that of Earth), is 95% CO2. The amount of CO2 in a column of the Martian atmosphere is about 17 times greater than in a similar column of Earth’s atmosphere. However, the collisional broadening of the CO2 absorption and emission lines in the thin Martian atmosphere is much less than on Earth. This factor, together with the lower temperatures on Mars, reduces the absorptivities and emissivities enough to make the CO2 atmospheric warming effect on Mars weaker than that on Earth. (The lack of water on Mars also means that the water vapor feedback that amplifies the CO2 atmospheric warming on Earth is not operative on Mars.) Exactly the opposite is the case for Venus with an atmosphere that is about 97% CO2 at a total pressure of 9300 kPa (more than 90 times that of Earth). The collisional broadening of the CO2 spectral lines is larger than on Earth and extends high into the Venusian atmosphere. This factor, and the higher temperature on Venus, increases the absorptivities and emissivities so much that Venus is sometimes characterized as having a runaway greenhouse effect that makes the Venusian surface temperature about 500 K higher than it would be without the atmospheric warming.