By Keith Michael Krise, Ph.D. and Scott A. Williams, Ph.D.
In the early days of photography, people used much simpler cameras. These cameras could “capture” images and focus their light onto light-sensitive material called photographic film. To take a picture, a photographer would focus their camera lens and press a button. The lens would then open for a short time, and snap shut. The amount of time the lens stayed open would depend on how much light was available. A picture taken on a cloudy day would need a longer exposure than a bright, sunny day.
The moment the picture was taken, the film had to be kept in total darkness inside the camera. To take a new picture, the photographer would use a fresh section of film. The film was a long roll, and a photographer could expose many different frames before they needed to load new film into the camera.
What was on the film that made it photosensitive? This is where we get to see some interesting chemistry at work! In black-and-white photography, film was made by coating it with gelatin (the same ingredient found in Jell-O desserts). This kind of gelatin contained silver bromide (AgBr for short). This is a simple chemical like the table salt (sodium chloride, NaCl) that you have in your kitchen, but it contains silver instead of sodium. The silver in silver bromide has a positive charge, and the bromide has a negative charge. The opposite charges on the silver and bromide ions cause them to be held together by an electrostatic force.
When light strikes the silver bromide, it causes the negative charge on the bromide to transfer to the positive silver. Since a negative charge cancels a positive charge, this makes the silver neutral. While the positively charged silver ion is transparent, and allows light to pass through the film, the neutral silver is opaque, meaning it blocks light. This happens all over the frame of film, making a pattern of light and dark in the form of the image captured with the camera.
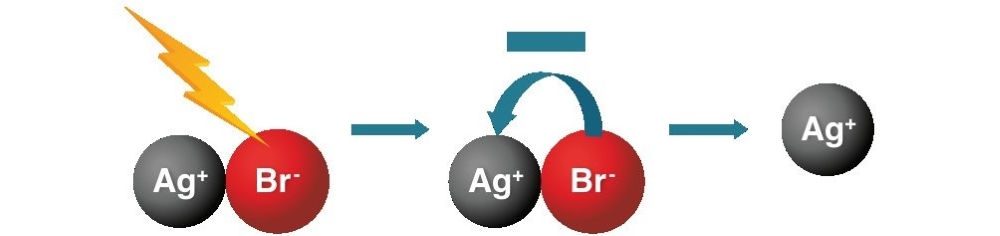
If the exposed film stays in the dark, any image on it is stable and will not change. But we don’t want to look at film only in the dark, so the film needs to be “fixed,” or locked in place. To do this, the photographer removed the film from the camera in a darkroom. Then they washed the film in a fixer solution that changed the positive silver ions remaining on the film into a form that was no longer sensitive to light. The image in the film was the reverse (or ‘negative’) of the actual scene captured.
The next step in the process was to shine light through the negative onto a piece of photographic print paper that was also light-sensitive. After this exposure, the photographic print had to be developed (using more chemistry). This process could take hours or even days because most people would send their film to a lab to have it created or developed and printed. It wasn’t until photos were developed that you knew whether you had taken a good picture or not. It should make you appreciate how fast and easy today’s digital photography works!
Keith Michael Krise, Ph.D. is Professor of Chemistry and Biochemistry at Gannon University. Scott A. Williams, Ph.D. is Professor of Inorganic Chemistry and Director of Materials Science and Engineering Graduate Program at Rochester Institute of Technology.


