What molecule am I?

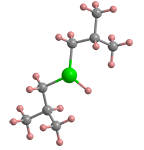
Diisobutylaluminum hydride (DIBAL-H) is an organometallic compound that has found wide use as a reducing agent. The molecule’s nominal structure is shown in the 3-D image: but under ambient conditions, it exists as a dimer, as illustrated in the 2-D image.
DIBAL-H first appeared in the chemical literature in 1955, when Karl Ziegler*1, Kurt Schneider, and Josef Schneider at the Max Planck Institute for Coal Research (Mülheim an der Ruhr, Germany) showed how it and other organoaluminum hydrides can be used to reduce aldehydes to alcohols. Since that time, DIBAL-H has been used under milder conditions to reduce carboxylic acids, esters, and nitriles to aldehydes. Under comparable conditions, former Molecule of the Week lithium aluminum hydride is a stronger reducing agent than DIBAL-H.
Like most metal hydrides, DIBAL-H oxidizes spontaneously in air and reacts violently, sometimes explosively, with water. Because of these hazards, it is most often sold as a solution in aliphatic hydrocarbons (e.g., hexane), aromatic hydrocarbons (e.g., toluene), or ethers (e.g., tetrahydrofuran).
1. Ziegler won a share of the 1963 Nobel Prize in chemistry for his research on polymers.
Diisobutylaluminum hydride hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Pyrophoric liquids, category 1 | H250—Catches fire spontaneously if exposed to air | |
| Chemicals that, in contact with water, emit flammable gases, category 1 | H260—In contact with water, releases flammable gases that may ignite spontaneously | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Serious eye damage, category 1 | H318—Causes serious eye damage | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the Journals
Picrotoxinin1 is one of two toxic compounds found in the fruit of the southeast Asian climbing plant Anamirta cocculus; the other is picrotin2. The combination, known as picrotoxin, has been known since 1812. Historically, the principal use of picrotoxin in A. cocculus seeds has been to stun fish and make them easier to catch.
The literature contains several syntheses of picrotoxinin; one of the more recent (2020) was reported by Ryan Shenvi and co-workers at the Scripps Research Institute (La Jolla, CA), who shortened the process to 13 steps by improving the formation of the molecule’s [4.3.0] bicyclic core. Last December, Shenvi’s group and colleagues at Oberlin College (OH) and Corteva Agriscience (Indianapolis) went on to improve the stability of picrotoxinin by methylating it at the C5 position, making it easier for researchers to use in drug-development studies.
Sodium pyruvate3 (NaPyr) and its conjugate, pyruvic acid4, are well-known biomolecules that provide energy to cells, protect them against hydrogen peroxide, and function as intermediates in several cellular metabolic processes. But this June, Jessica M. Weber and colleagues at the NASA Jet Propulsion Laboratory of Caltech (Pasadena, CA) showed that it may also have important implications for outer space exploration.
The researchers conducted laboratory simulations of the behavior of NaPyr on Ceres, the largest body in the asteroid belt and the nonterrestrial body richest in water, to develop an understanding of how the important proto-metabolic substance would function on the surface of the dwarf planet. In studies of NaPyr under conditions relevant to Ceres’s surface, they found that decarboxylation reactivity differed depending on whether NaPyr was isolated or in contact with minerals on the body’s surface and that NaPyr reactivity occurred only under irradiation with UV light.
1. CAS Reg. No. 17617-45-7.
2. CAS Reg. No. 21416-53-5.
3. CAS Reg. No. 113-24-6.
4. CAS Reg. No. 127-17-3.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Diisobutylaluminum
hydride fast facts
| CAS Reg. No. | 1191-15-7 |
| SciFindern name | Aluminum, hydrobis(2-methylpropyl)- |
| Empirical formula | C8H19Al |
| Molar mass | 142.22 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 116–118 °C (1 torr) |
| Water solubility | Reacts violently |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

