What molecule am I?
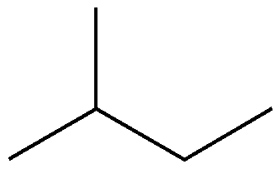
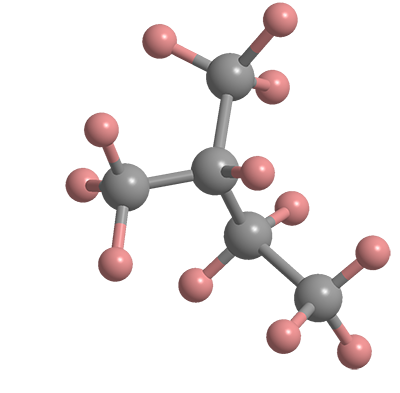
2-Methylbutane, commonly known as isopentane, is a branched-chain, saturated hydrocarbon. It has two structural isomers, pentane and neopentane.
In 1884, pioneering British organic chemist William Henry Perkin1 used 2-methylbutane as a test molecule in his treatise “On the magnetic rotary polarization2 of compounds in relation to their chemical constitution”.
Branched alkanes constitute the largest group of molecules in gasoline (25–40%); 2-methylbutane is one of the most abundant in that group. Other than gasoline, the compound has few commercial uses; the most predominant is as a component of blowing agents for phenolic resins and polyurethane foams. A 2024 Chinese patent includes 2-methylbutane as a component of lead-free aviation gasoline.
1. Perkin famously (and serendipitously) discovered the purple dye mauveine at age 18.
2. Also known as the Faraday effect, magnetic rotary polarization occurs when a magnetic field alters the electromagnetic properties of a material so that it causes polarized light to rotate.
2-Methylbutane hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 1 | H224—Extremely flammable liquid and vapor | |
| Aspiration hazard, category 1 | H304—May be fatal if swallowed and enters airways | |
| Specific target organ toxicity, single exposure, central nervous system, category 3 | H336—May cause drowsiness or dizziness | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Difluoromethyl nitrile oxide1 (CHF2CNO) is an organic reagent that has come into recent use for functionalizing unsaturated substrates. It first appeared in the chemical literature in 1982, when Christopher Glidewell* and H. Diane Holden at the University of St. Andrews (UK) included it in a theoretical study of 67 pseudohalides.
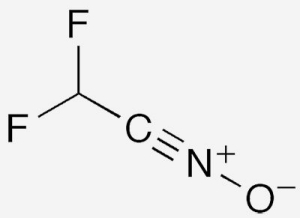
In 2017, CHF2CNO reappeared in a paper by Pavel K. Mykhailiuk and colleagues at Taras Shevchenko National University of Kyiv (Ukraine) and Enamine Ltd./Bienta (Kyiv), who described it as a “neglected chemical reagent”. In a cycloaddition reaction with alkynes or enamines, they prepared difluoromethyl-substituted isoxazoles, which they believed would be promising core groups in new agrochemicals. Then, this past March, Mykhailiuk and his Enamine team reported that CHF2CNO reacts with a wide range of alkenes to form difluoromethylisoxazolines. In both cases, CHF2CNO is generated in situ, and the reactions proceed at ambient temperature.
1. CAS Reg. No. 83620-10-4.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
2-Methylbutane
fast facts
| CAS Reg. No. | 78-78-4 |
| SciFindern name | Butane, 2-methyl- |
| Empirical formula | C5H12 |
| Molar mass | 72.15 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 28 °C |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

