What molecule am I?
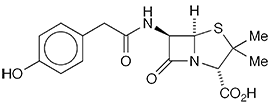
Penicillin X1, also known as penicillin III, is a hydroxybenzyl derivative of former Molecule of the Week benzylpenicillin2 (penicillin G or II). These and other penicillin derivatives were developed during World War II and saved as many as 100,000 lives. Penicillins are β-lactam antibiotics that are formed in molds of the genus Penicillium,
Penicillin G is the standard against which other penicillins are compared for potency and other characteristics. In a 1946 study by Harry Eagle at the Johns Hopkins School of Hygiene (Baltimore), penicillin X was 30–40% more potent than G against certain Streptococcus and Spirochetes bacteria in vitro.
The same year, Gladys L. Hobby, Blanche Burkhart, and Beverly Hyman at Pfizer (then in Brooklyn, NY) compared penicillins F3, K4, and X with G (the four characterized penicillins at the time) in terms of their relative activity against streptococcal infections in mice. Depending on the basis used for comparison, X was 3–5 times more efficacious than G, whereas F and K were slightly less and significantly less efficacious, respectively.
These results and others beg the question: Why is penicillin G, and not X, the only one in use today? Your editor will leave it to readers to respond to this question.
1. SciFindern name: 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-{[2-(4-hydroxyphenyl)acetyl]amino}-3,3-dimethyl-7-oxo-, (2S,5R,6R)-.
2. CAS Reg. No. 61-33-6.
3. CAS Reg. No. 118-53-6.
4. CAS Reg. No. 525-97-3.
Penicillin X hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Respiratory sensitization, category 1 | H334—May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
*Data for benzylpenicillin; No data available for penicillin X.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update
Hydrogen sulfide1 (H2S) was the Molecule of the Week for July 6, 2015. It is a noxious and poisonous gas with a “rotten egg” odor. It has some industrial uses, but recently its medicinal activity has been explored as well.
According to Philip J. Milner and colleagues at Cornell University (Ithaca, NY), Southern Methodist University (Dallas), and the Korea Institute of Science and Technology (Seoul), “Hydrogen sulfide . . . is an endogenously produced gasotransmitter involved in many physiological processes that are integral to proper cellular functioning. . . . [It] plays important roles in preventing inflammatory skin disorders and improving wound healing.”
Transdermal delivery is a viable method for delivering H2S to patients, but it can generate toxic byproducts. Earlier this month, the authors reported that encapsulating H2S in metal–organic frameworks with coordinatively unsaturated metal centers allows the gas to be delivered to porcine skin with greater efficiency and sustainability than H2S alone and with no toxic effects.
1. CAS Reg. No. 7783-06-4.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Penicillin X
fast facts
| CAS Reg. No. | 525-91-7 |
| Empirical formula | C16H18N2O5S |
| Molar mass | 350.39 g/mol |
| Appearance | White crystals or powder |
| Melting point | 82–83 °Ca |
| Water solubility | 210 mg/La |
a. Data for benzylpenicillin (penicillin G). No data available for penicillin X.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

