What molecule am I?

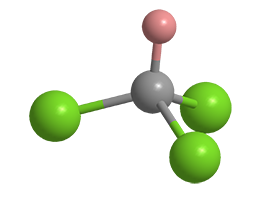
Chloroform (CHCl3) is a solvent, reagent, refrigerant, and anesthetic with a chemical history that dates back to the 1830s. During that period, at least five scientists synthesized it; but there was much confusion about its chemical formula. Proposed formulas ranged from CH2ClCH2Cl (“chloric ether” or 1,2-dichloroethane) to C2Cl5 (an impossibility) to C2H2Cl6 (double the actual formula).
By the 1840s, chloroform began to be used as an anesthetic; and by the next decade, it was manufactured on a large scale via the reaction between chloral1 and sodium hydroxide. Chloroform was produced by this process for more than a century; after which, it was replaced by heating methane and/or chloromethane with chlorine. This method produces dichloromethane as well.
An early use of chloroform as a solvent appeared in an 1882 article by Arthur H. Elliott at Columbia College (now Columbia University, New York City). Elliott assessed the solubility of then-new “nitrosaccharose” (nitrocellulose2) in about 40 solvents available to him and found that chloroform dissolved the substance at ambient temperature. Chloroform eventually became a widely used industrial and laboratory solvent until recent decades when its hazardous properties (see hazard information table) caused regulatory agencies to restrict its use.
Chloroform alone has little use as a refrigerant; but large quantities of it were used to synthesize chlorodifluoromethane3 (CHClF2), a widely used refrigerant until it was phased out because of its high ozone-depleting and global-warming potentials. Besides CHClF2 synthesis, chloroform, when treated with sodium hydroxide, is used to generate dichlorocarbene4 (:CCl2), which reacts with alkenes in situ to produce dichlorocyclopropane derivatives.
The global chloroform market in 2022 was ≈3.8 million tonnes.
1. CAS Reg. No. 75-87-6.
2. CAS Reg. No. 9004-70-0.
3. CAS Reg. No. 75-45-6.
4. CAS Reg. No. 1605-72-7.
Chloroform hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ toxicity, single exposure, narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
| Specific target organ toxicity, repeated exposure, oral, category 1 | H372—Causes damage to organs (liver, kidney) through prolonged or repeated exposure if swallowed | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Xanomeline1 is a muscarinic acetylcholine receptor agonist that was originally developed in the 1990s as a possible Alzheimer's disease treatment. Trospium chloride2 is a 1970s-era muscarinic antagonist used to treat overactive bladder. How are these two drugs connected?
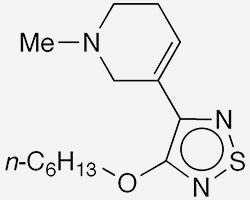
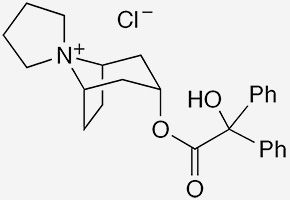
During trials on xanomeline, Neil C. Bodick and colleagues at Eli Lilly (Indianapolis) and several other institutions observed that the drug alleviated psychotic symptoms associated with Alzheimer’s. But Lilly shelved xanomeline research because of its gastrointestinal side effects.
In 2012, Andrew Miller, recognizing that xanomeline still had promise, licensed it from Lilly to start a new company, Karuna Therapeutics (Boston), for developing it to treat schizophrenia. To combat the gastrointestinal problems, Steven M. Paul and co-workers at Karuna combined it with trospium chloride, which does not cross the blood–brain barrier but blocks muscarinic receptors in the gut. The drug combination, called KarXT, has undergone clinical trials and was submitted for approval by the US Food and Drug Administration last November.
1. CAS Reg. No. 131986-45-3.
2. CAS Reg. No. 10405-02-4.
Molecules of the Future
Once a month we bring you a newly discovered or developed molecule(s) that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecules of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Chloroform fast facts
| CAS Reg. No. | 67-66-3 |
| SciFindern name | Methane, trichloro- |
| Empirical formula | CHCl3 |
| Molar mass | 119.38 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 61 °C |
| Water solubility | 8 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

